What Is An N-Type Semiconductor?
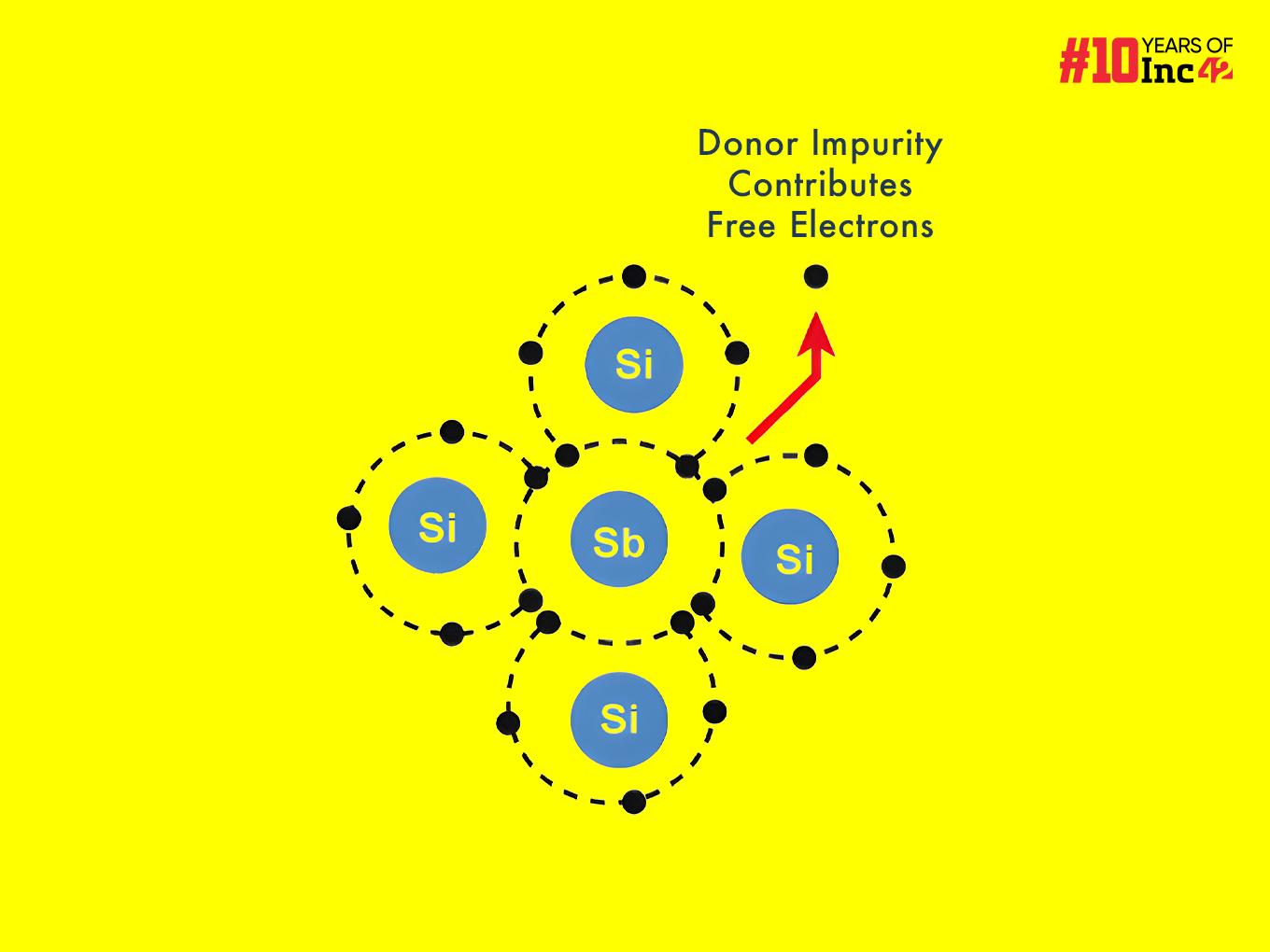
An n-type semiconductor is a type of semiconductor material that shows increased conductivity when donor impurities are introduced. These donor atoms are typically elements from Group V of the periodic table, like phosphorus (P), arsenic (As) or antimony (Sb), and have five valence electrons in their outer shell.
When introduced into the crystal lattice of a Group IV semiconductor like silicon (Si) or germanium (Ge), which possess four valence electrons, one of the donor atom’s five valence electrons forms covalent bonds with the four neighbouring semiconductor electrons. However, the fifth valence electron from the donor atom remains loosely bound to its nucleus. It can be easily excited into the conduction band, where it can participate in electrical conduction.
This additional electron population in the conduction band contributes to the overall conductivity of the n-type semiconductor.
How Is An N-Type Semiconductor Created?
An n-type semiconductor is created through doping, where a carefully controlled amount of impurity atoms are introduced into a pure semiconductor crystal. The following is a general breakdown of the steps involved:
- Starting Material: The process begins with a pure semiconductor, typically silicon (Si) or germanium (Ge). These elements have four valence electrons in their outer shell, allowing them to form a stable crystal structure with covalent bonds. However, in their pure state, they have very low conductivity.
- Doping With Donor Atoms: Elements from Group V of the periodic table are used as dopants. These elements have five valence electrons in the outer shells of their atoms.
- Integration Into Crystal Lattice: During the manufacturing process, a tiny amount of the donor element is introduced into molten silicon or germanium. As the material cools and solidifies, the donor atoms become integrated into the semiconductor crystal lattice.
- Creating Free Electrons: Each donor atom forms covalent bonds with four neighbouring silicon or germanium atoms using its four outer electrons. However, the fifth valence electron from the donor atom does not participate in bonding and remains loosely bound to the nucleus. This electron requires minimal energy to be excited into the semiconductor’s conduction band.
- Increased Conductivity: With the introduction of donor atoms, there is a significant increase in the number of free electrons within the conduction band. These additional electrons are the majority charge carriers in n-type semiconductors and contribute to their enhanced conductivity compared to pure silicon or germanium.
Group V Elements & N-Type Semiconductors
Group V elements play a crucial role in creating n-type semiconductors. Here’s the connection, explained from a more technical perspective:
- Electronic Structure: Elements in Group V of the periodic table, such as phosphorus, arsenic, and antimony, possess a specific electronic configuration. Their atoms’ outer shells hold five valence electrons and their valence shell electron configuration is defined as ns²np³ orbital notation (where n is the principal quantum number). This contrasts with Group IV elements like silicon and germanium, which have a notation of electrons ns²np² configuration.
- Doping & Lattice Substitution: A small amount of a Group V element is introduced into the intrinsic silicon or germanium crystal lattice during doping. These dopant atoms substitute a silicon/germanium atom within the lattice structure.
- Donor Impurity Levels: The key aspect lies in the dopant’s electronic structure. Four of the five valence electrons from the Group V element participate in covalent bond formation with the four nearest neighbouring silicon/germanium atoms, replicating the bonding scheme of the original lattice.
However, the fifth valence electrons (ns² orbital) of the Group V element experience weaker attraction to its nucleus as compared to the other four electrons. This creates a donor impurity level within the semiconductor bandgap, positioned slightly below the conduction band minimum.
- Thermal Excitation & Free Electrons: Due to the lower energy state of the donor level compared to the conduction band, thermal energy at room temperature is sufficient to excite a significant number of electrons from the donor level into the conduction band.
The loosely bound fifth electron readily absorbs thermal energy and transitions to a mobile state within the conduction band. This population of excited electrons becomes the majority charge carriers in the n-type semiconductor.
- N-Type Characterisation: With an abundance of freely moving electrons as the dominant charge carriers, the doped semiconductor is categorised as an n-type semiconductor. The negative charge carriers (electrons) contribute significantly to electrical conduction within the material.
How Are N-Type Semiconductors Used In Electronics?
N-type semiconductors are fundamental building blocks in a range of electronic devices. Here are some prominent applications:
- Transistors: These workhorse devices are the foundation of modern electronics. N-type semiconductors are used in bipolar junction transistors (BJTs) and field-effect transistors (FETs). In BJTs, they typically form the emitter and collector regions, controlling the flow of current through the device. In FETs, such as MOSFETs, n-type semiconductors make up the source and drain regions, responsible for carrying the current.
- Diodes: N-type semiconductors play a crucial role in p-n junction diodes. The p-n junction is formed by creating a region of p-type (with positive charge carriers called holes) next to an n-type region within the same semiconductor material. This creates a unique electrical behaviour where current can flow readily in one direction but is blocked in the other. Diodes are used in various applications like rectification (converting AC to DC), voltage regulation, and signal processing.
- Integrated Circuits (ICs): These complex miniaturised circuits contain billions of transistors and other electronic components. N-type and p-type semiconductors are strategically arranged to create various logic gates, memory elements, and other functional blocks that form the core of an IC.
- Solar Cells: N-type semiconductors play a vital role in converting sunlight into electricity. In a typical solar cell, a p-n junction is formed, with the n-type layer absorbing a significant portion of the light. When sunlight strikes the cell, it excites electrons, creating an electric current that can be harnessed for power.
- Light-Emitting Diodes (LEDs): LEDs emit light when an electric current flows through them. N-type semiconductors can be used in certain LED designs with p-type regions to facilitate the recombination of electrons and holes, leading to light emission.